The current industrial ammonia synthesis technology is mainly based on the Haber-Bosch method using iron-based catalysts. Its reaction conditions are very harsh (250 atm, 400 degrees Celsius) and require huge energy consumption. Photocatalysis technology can directly convert solar energy into chemical energy, which provides a very promising method for reducing the energy consumption of synthetic ammonia. However, the ultra-high bond energy of the nitrogen-nitrogen triple bond makes nitrogen molecules exhibit stable chemical properties, which makes it difficult for conventional photocatalytic materials to activate nitrogen molecules. Therefore, the development of efficient nitrogen-fixing ammonia synthesis photocatalysts still faces great challenges. Recently, the team of Professor Xiong Yujie of the University of Science and Technology of China and Professor Wu Xiaojun's theoretical research team cooperated to control the defect engineering of metal oxide photocatalysts. It was found that refining the defect state of the catalyst by doping can promote the defect site to nitrogen molecules The high-efficiency activation of TiO2 effectively improves the efficiency of photocatalytic nitrogen fixation to ammonia. The work was published online in the International Journal of Chemistry, American Journal of Chemistry (J. Am. Chem. Soc. DOI: 10.1021 / jacs.8b02076). The co-first authors are Dr. Zhang Ning, PhD students Abdul Jalil and Wu Daoxiong, Co-corresponding authors include Professor Xiong Yujie, Gao Chao's special associate researcher and Professor Wu Xiaojun. From a kinetic point of view, in view of the ultra-high chemical stability of nitrogen molecules, activation of nitrogen molecules is generally regarded as a prerequisite for nitrogen reduction. For photocatalytic materials, the surface defect site can be used as the active site for the chemical adsorption of nitrogen molecules, and the electrons localized at the defect can be transferred into the reverse bond π orbital of the adsorbed nitrogen molecule, thereby weakening the nitrogen-nitrogen triple bond effect. Although there have been reports in the related literature that the catalyst material based on the defect structure can be used in the photocatalytic nitrogen-fixing ammonia synthesis reaction, its activity still needs to be further improved. The bottleneck comes from many aspects: firstly, it is necessary to further regulate the adsorption of nitrogen molecules by the catalytic site to promote the transfer of photogenerated electrons from the catalyst to the adsorbed nitrogen molecules, so as to improve the activation ability of the nitrogen molecules; Energy relaxation process to reduce energy loss during electron transfer. In response to this series of challenges, the team of Xiong Yujie doped molybdenum atoms at the defect sites of the W18O49 catalyst to achieve efficient activation of nitrogen molecules in the photocatalytic system. The researchers combined the characterization of synchrotron radiation technology, in-situ infrared spectroscopy detection and theoretical calculation simulation to reveal the refined effect of doped molybdenum atoms on the defect state. On the one hand, molybdenum doping enhances the defect level of the catalyst and reduces the energy loss caused by the relaxation process of electronic energy; on the other hand, the molybdenum-tungsten heterosites formed by the molybdenum doping control the charge state of nitrogen molecules. , Increases the charge difference between nitrogen atoms, and at the same time improves the covalentity of the metal-oxygen bond, and promotes the photogenerated electron transfer process. The synergy between the different effects brought about by these molybdenum dopings effectively promotes the activation of nitrogen molecules at the catalytic site, and achieves a significant increase in the efficiency of catalyst light-driven nitrogen fixation to ammonia synthesis. This progress provides a new idea for the development of high-efficiency nitrogen-fixing photocatalysts and the regulation of catalyst defect states, and shows the importance of the regulation of the electronic structure of the catalytic site on the catalytic reaction. The synchrotron radiation X-ray absorption spectrum characterization, photoelectron energy spectrum characterization and infrared spectroscopy in-situ detection of this work were supported by the cooperation of Professor Song Li, Professor Zhu Junfa and Associate Researcher Qi Zeming, University of Science and Technology of China, respectively. The research work was supported by projects such as the National Key R & D Program, the National Outstanding Youth Science Fund, the Chinese Academy of Sciences Frontier Science Key Research Projects, and the Chinese Academy of Sciences Innovation Cross Team.
The distributor chute is a kind of wearing parts , which serves a very short working time, mostly maintain 6 to 12 months. Under high temprature working condition, the anti-abrasion performance of metallic material will be degraded. Take 850℃ working temprature and impact property into account, Hard-plate adopts HP300 welding wire to satisfy blast furnace severe condition.
High Temperature Distributor Chute High Temperature Distributor Chute,Chromium Carbide Hardfacing Overlay,Blast Furnace Distributor Chute,Chromium Carbide Distributor Chute SHENYANG HARD WELDING SURFACE ENGINEERING CO.,LTD , https://www.hardfacingplate.com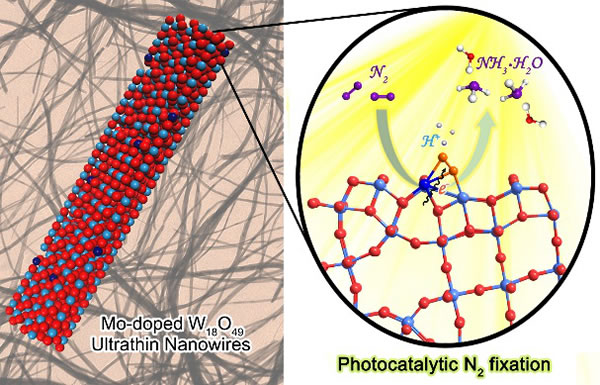
Schematic diagram of W18O49 catalyst based on molybdenum-doped refined defect states for light-driven nitrogen-fixing ammonia synthesis
China University of Science and Technology has made new progress in the development of a photocatalytic nitrogen-fixing ammonia synthesis catalyst
